A light-induced transfection system (LITS) was designed to precisely control the delivery of mRNA, achieving spatiotemporal de-coupling of the pleotropic inflammatory and tolerogenic signals of interleukin-2 to conduct safe eradication of tumors.
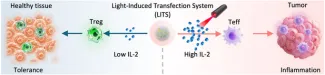
Spatiotemporal control of protein expression remains a critical challenge in messenger RNA (mRNA) therapeutics, particularly for tumor‐targeted therapy. Here, we introduce a Light‐Induced Transfection System (LITS) leveraging photosensitizing polymers to deliver mRNA systemically and activate its translation via light‐triggered endosomal escape. This system enables localized protein expression in any irradiated tissue, allowing for spatial control of therapeutic effects. Using interleukin‐2 (IL‐2) as a model, we demonstrate that LITS can trigger proinflammatory cytokine levels in irradiated tumors, while inducing tolerogenic IL‐2 levels in nonirradiated healthy tissues. This modulation of IL‐2′s pleiotropic effects provides both potent antitumor activity and reduced toxicity. Furthermore, by optimizing light exposure, LITS synergizes IL‐2 efficacy by driving immunogenic cell death to eradicate lung metastasis in a breast cancer model. Our findings establish LITS as a programmable mRNA delivery platform for on‐demand targeted and safe therapies.