Abstract
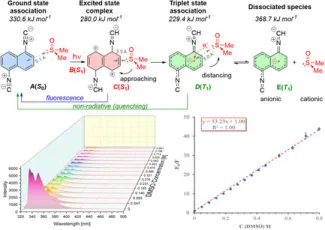
Detecting dimethyl sulfoxide (DMSO) is important across chemical and environmental contexts because of its toxicity concerns and effects on cells. Fluorometry offers a rapid, sensitive readout, yet many dyes are scarce or synthetically complex. In this proof-of-concept study, we present a cheap and effective probe, namely 1,5-diisocyanonaphthalene (1,5-DIN), able to detect sulfoxides (dimethyl sulfoxide, DMSO and tetramethyl sulfoxide, TMSO) in various organic (MeCN, THF, MeOH, iPrOH, EtOAc) and aqueous media over the industrially and environmentally relevant range of 0.005–0.8 M (0.03-5% v/v). With a molecular weight of only 178.2 g mol−1, this is the lowest molecular weight fluorescent probe for the purpose. The limit of detection (LOD) was 5 and 18 ppm for DMSO in MeCN and water, respectively. It was established that LOD values increase in protic solvents due to H-bond formation between the solvent and DMSO. Our detailed quantum chemical calculations revealed that upon photoexcitation the electron-deficient aromatic ring of DIN attracts DMSO, forming a stable complex. Quenching is explained by the proximity of the S1 minimum to the T1 triplet curve, allowing intersystem crossing and enabling a nonradiative de-excitation process through electron transfer from 1,5-DIN back to DMSO.
Graphical Abstract
We present 1,5-DIN as a low-cost, efficient fluorescent probe for trace DMSO detection in organic and aqueous media. It shows strong quenching, with LODs from 5–50 ppm, even in complex matrices. Theoretical insights support its mechanism, highlighting its promise for practical applications.